Introduction: SystemicAL amyloidosis is a progressive and often fatal disease caused by misfolded light chains that aggregate into amyloid fibrils and deposit in vital organs, leading to organ dysfunction. Current standard of care (SoC) therapies for AL amyloidosis target the plasma cell clone to reduce or eliminate light chain production, but do not remove soluble toxic light chain aggregates or insoluble amyloid deposits. Early mortality remains high for patients with advanced disease (e.g., Mayo 2012 Stage IV disease, median overall survival <6 months). Birtamimab - a humanized monoclonal antibody that neutralizes soluble, toxic light chain aggregates and depletes insoluble amyloid deposits by inducing phagocytosis - is under investigation for the treatment of AL amyloidosis. Here, we present a pooled safety analysis from previous Phase 1-3 clinical trials, with a focus on the 2 double-blind randomized controlled trials (RCTs): PRONTO (NCT02632786) and VITAL (NCT02312206).
Methods: Patients with AL amyloidosis were treated with intravenous birtamimab up to 24 mg/kg every 28 daysin the non-placebo-controlled Phase 1/2 open-label trial (NCT01707264) with open-label extension (OLE; NCT02613182) and 2 RCTs (Phase 2 PRONTO with OLE [NCT03154047] and Phase 3 VITAL). In PRONTO, patients had received ≥1 previous systemic therapy with at least a partial hematologic response and persistent cardiac dysfunction, and received birtamimab or placebo. In VITAL, newly diagnosed treatment-naïve patients with cardiac involvement received birtamimab + SoC or placebo + SoC (where SoC was a bortezomib-containing chemotherapy regimen). The analysis included all patients who received ≥1 dose of birtamimab. Pooled median exposure times were calculated, and baseline characteristics and demographics were summarized. For VITAL and PRONTO, pooled rates and severity of treatment-emergent adverse events (TEAEs) with birtamimab were compared with rates in the placebo arms. In addition, safety data from the non-placebo-controlled studies were summarized.
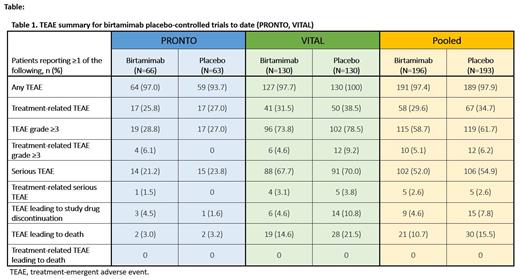
Results: The median (range) exposure to birtamimab among 302 patients pooled from the Phase 1-3 trials was 12.24 (0.03-57.72) months. Of the 302 patients exposed to birtamimab, 295 received at least one 24-mg/kg infusion, up to 2500 mg. Analysis of the 2 randomized, placebo-controlled trials included data from 196 patients treated with birtamimab from the PRONTO (n=66) and VITAL (n=130) trials and 193 patients who received placebo (PRONTO, n=63; VITAL, n=130). Both trials had comparable treatment durations overall. The rates of TEAEs, serious and grade ≥3 TEAEs were similar between birtamimab and placebo arms within each trial and between treatment arms in the placebo-controlled pooled analysis set (PRONTO and VITAL; Table 1). Higher rates of treatment-related, serious, and grade ≥3 TEAEs were reported in VITAL compared with PRONTO, presumably due to the treatment-naïve population and receipt of concomitant SoC therapy in VITAL. In the pooled analysis set, the most common TEAEs that occurred with greater frequency in the birtamimab vs placebo arms in either trial were, respectively, fatigue (36.2%, 34.2%), diarrhea (33.2%, 33.7%), nausea (31.6%, 30.1%), constipation (31.1%, 31.6%), and dyspnea (25.0%, 24.9%). The most commonly reported (≥5%) serious and grade ≥3 TEAEs were primarily cardiac disorders (cardiac failure, syncope, cardiac arrest), consistent with the underlying disease. Infusion-associated TEAE rates were low in the pooled birtamimab and placebo arms (5.1% vs 3.6%, respectively) and were generally mild to moderate. The safety profile observed in the non-placebo-controlled Phase 1/2 trial plus OLE and PRONTO OLE (n=106) was consistent with the safety data reported in the placebo-controlled studies.
Conclusions: Birtamimab was well tolerated in patients with AL amyloidosis when administered as monotherapy and did not demonstrate additive toxicity when given with SoC chemotherapy. Rates of TEAEs in the birtamimab arm were similar to rates in the placebo arm in the PRONTO and VITAL trials. The safety and efficacy of birtamimab is being further investigated in an ongoing confirmatory Phase 3 double-blind RCT in patients with Mayo Stage IV AL amyloidosis (AFFIRM-AL; NCT04973137), which is currently enrolling.
Disclosures
Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wechalekar:Alexion/AstraZeneca, Attralus, GSK, Janssen, Pfizer, Prothena: Consultancy, Honoraria. Kastritis:Sanofi: Honoraria; Pfizer: Honoraria, Research Funding; GSK: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Palladini:Siemens: Honoraria; Sebia: Honoraria; Prothena: Consultancy, Honoraria; Pfizer: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Argobio, GSK: Consultancy. Schönland:Janssen, Takeda, Pfizer, Prothena: Honoraria; Prothena, Janssen, Sanofi: Research Funding; Janssen, Prothena, Celgene, Binding Site, Jazz: Other: Travel grant. D'Souza:Imbrium, Pfizer, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen, Prothena: Consultancy; Abbvie, Sanofi, Takeda, TeneoBio, Caelum, Prothena: Research Funding. Khouri:GPCR Therapeutics: Honoraria; Janssen: Consultancy, Honoraria. Jimenez-Zepeda:Janssen, Prothena, BMS, Angen,: Honoraria. Sprinz:Prothena Biosciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Hao:Prothena Biosciences: Current Employment, Current equity holder in publicly-traded company. Huang:Prothena Biosciences: Current Employment, Current equity holder in publicly-traded company. Aubrey:Prothena Biosciences: Current Employment, Current equity holder in publicly-traded company. Gertz:Ionis/Akcea, Prothena, Sanofi, Janssen, Aptitude Healthgrants, Ashfield, Physicians Education Resource, Research to Practice, Johnson & Johnson, and Celgene: Consultancy; AbbVie: Other: Data Safety Monitoring board; i3HEalth: Other: For development of educational material; Juno Pharmaceutics, and Sorrento Therapeutics: Other: Meetings.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal